Development and Characterization of a Mouse Model of Immuno-Aging
The hematopoietic stem cell (HSC) is maintained in the adult bone marrow by complex networks of stromal cells as well as HSC progenies, which control HSC self-renewal and differentiation. The balanced production of all blood components is crucial for lifelong adaptation to infectious and environmental challenges and resilience of the entire organism. Skewing of hematopoiesis to myeloid lineages at the expense of lymphocytic differentiation is a hallmark of aging. This imbalance of blood cell production is considered a major contributor to age-related diseases ranging from cancer and cardiovascular failure to chronic inflammation and impaired adaptive immune responses. We identified a mutant mouse model with accelerated age-related myeloid skewing that persists in HSC transplanted into young mice. This observation allows us to generate large numbers of mice in which HSC prematurely age in otherwise young organisms. With these mice we have a unique mouse model of HSC-dependent immuno-aging to advance the central ReALity topic how system specific decline in aging and interventions targeting HSC influence other systems.
The investigators of this project will thoroughly characterize the bone marrow niche and hematopoietic cell composition of this mouse model and establish the response to different vaccination strategies as an initial proof of concept for defects in immuno-aging. The versatility of generating cohorts of mice with a prematurely aged hematopoietic system will allow us to provide mice, tissues and cells for functional and omics analysis to other investigators in ReALity.
Principal Investigators
Prof. Dr. Wolfram Ruf, University Medical Center Mainz (contact person, e-mail)
Dr. Borhane Guezguez, University Medical Center Mainz
Dr. med. Sebastian Kreiter, TRON
Dr. med. Michael Kühn, University Medical Center Mainz
Outcomes and Achievements of the Project:
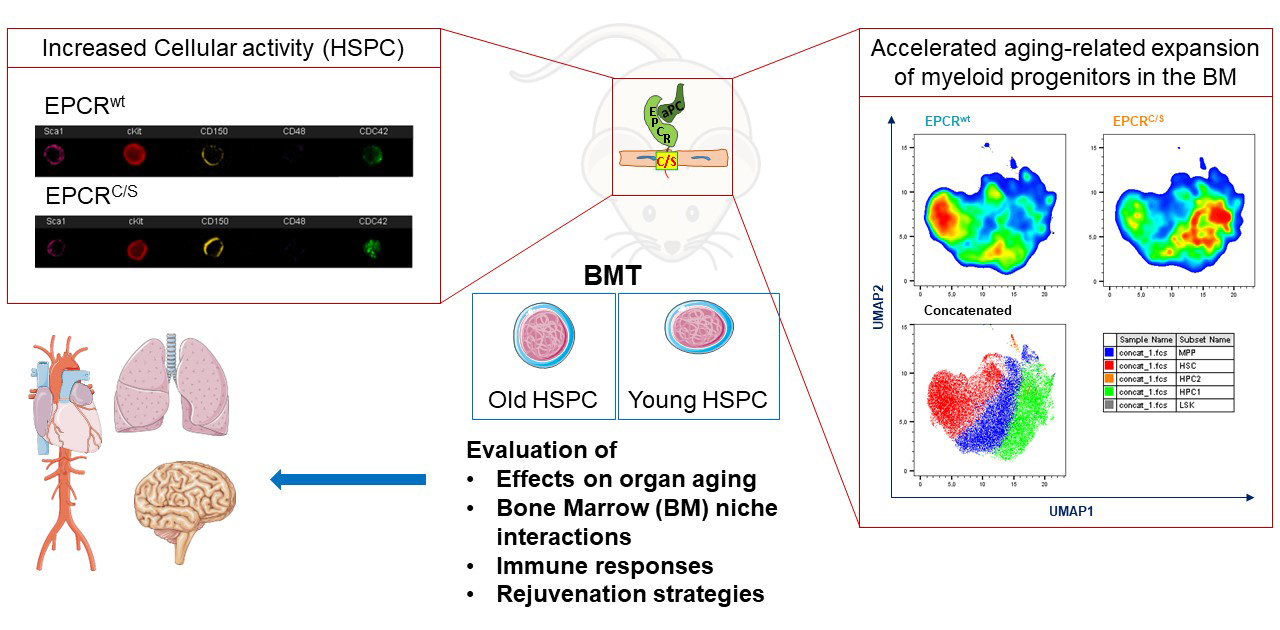
The aging phenotypes of mice was characterized with serially transplanted bone marrow (BM) from EPCRC/S versus strain-matched wild type (WT) mice (Fig. 1). EPCRC/S is a stem cell marker and coagulation protease receptor which was modified by targeted point mutation (intracellular Cys to Ser). The investigators found that EPCRC/S mice have a profound defect in anchoring the HSC to the extracellular matrix mediated by the integrin α4β1. This integrin controls the activity of the rho-like GTPase cdc42 which is hyperactivated in aging. The cdc42-dependent crosstalk between EPCR and integrin α4β1 was shown to be specifically disrupted in EPCRC/S mice. HSC and myeloid biased and committed progenitors showed increased cell cycle activity, an increased susceptibility to myeloid leukemia development, and decreased resilience to BM stress in EPCRC/S versus WT mice.
Moreover, the investigators developed new technologies to characterize the regulatory roles of coagulation and vascular signaling systems in the BM microenvironment (BMM) of aging mice. As one approach to better understand the spatial organization of the BMM during aging,the investigators focused on imaging the BM stromal architecture from matching young/old femurs. Whole-tissue imaging of bone sections was optimized for immunodetection of endothelial cells (CD31, endomucin), HSC (EPCR, CD150), and megakaryocytes (CD41). Imaging approaches were also developed to distinguish between platelet producing (ARNTL1+) and HSC niche supporting (MYLK4+) megakaryocytes for image quantification (Fig. 2).
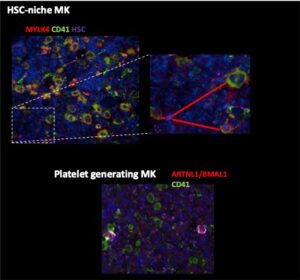
Fig. 2: Identification of ARNTL1/BMAL1 expressing platelet producing CD41+ megakaryocytes and MYL4/CD41+ HSC niche supporting megakaryocytes in the BM of mice.
In addition to high dimensional, multicolor flow cytometry the investigators developed single cell mRNA sequencing as a tool to precisely define phenotypic alteration in the hematopoietic stem and progenitor cells during normal and premature aging in the BM niche. The bioinformatic analysis clearly identifies the expansion of aged hematopoietic stem cells (Fig. 3). Established data sets from coagulation signaling altered young and old mice will allow the identification of cellular pathways for altered stem cell aging in these mouse models.
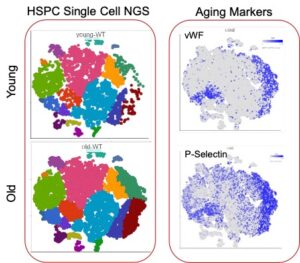
Fig. 3: Single cell sequencing analysis of hematopoietic stem and progenitor cells (LSK cells) isolated from the BM of adult and aged (>80 weeks) mice. In the combined data set the aging markers VWF and P-selectin clearly identify an expansion of phenotypically aged stem cells.
Summary and Outlook:
The project allowed the development and identification of strategies that combine multidimensional flow cytometry and sc-RNAseq for theinvestigation of phenotypical and transcriptional changes between young and aged hematopoietic stem and progenitor cells. Furthermore, the fine tuning of imaging approaches created the foundation for the establishment of further spatial analyses to characterize changes indistribution and localization of the different cell populations and sub-populations in the BMM during aging. We have accumulated a vast amount of data and established comprehensive technologies to study systemic mechanisms of aging with a focus on the BMM and stem cell exhaustion. We have established additional collaborations (Akilli-Öztürk group, TRON, and Gaida group, UMC/TRON) in spatial RNAmapping approaches of the BMM. The ultimate focus will be on the development of rejuvenation strategies.
Publications:
Winter S., Goetze KS., Hecker JS., Metzeler KH., Guezguez B., Woods K., Medyouf H., Schäffer A., Schmitz M., Wehner R., Glauche I., Roeder I., Rauner M., Hofbauer LC., Platzbecker U. Clonal hematopoiesis and its impact on the aging osteo-hematopoietic niche. Leukemia. 2024 May;38(5):936-946

